
TB Risk Assessment Tool
Personalized TB Risk Assessment
This tool estimates your risk of developing pulmonary tuberculosis based on key factors discussed in the article. It's designed to help you understand prevention options tailored to your situation.
Your Information
Risk Assessment Results
Enter your information to see your personalized risk assessment.
When you hear the term pulmonary tuberculosis, you probably picture a chronic cough and a long course of antibiotics. But the battle isn’t just about treating disease - it’s about stopping it before it starts. Over the next decade, prevention will shift from vague public‑health slogans to precise, science‑driven tools. Below you’ll find the roadmap that researchers, clinicians, and policy‑makers are following to make TB a thing of the past.
Key Takeaways
- New subunit vaccines (like M72/AS01E) could cut active TB cases by up to 50% in high‑risk groups.
- CRISPR‑based diagnostics promise results in under an hour, enabling rapid contact‑tracing.
- Digital adherence technologies are already improving completion rates for preventive therapy.
- Policy alignment with the WHO End TB Strategy remains the linchpin for scaling innovations.
- Patients can expect shorter preventive regimens and more personalized risk assessment.
Why Prevention Matters More Than Ever
In 2024, the World Health Organization reported roughly 10million new TB cases worldwide, with about 1.5million deaths. Roughly 85% of those infections are pulmonary, meaning they spread through the air and fuel the epidemic. Even though we have a century‑old vaccine - the BCG - its protection wanes after childhood and varies by geography.
Skipping prevention means relying on a cascade of diagnostics, lengthy drug regimens, and costly hospital stays. Each missed case adds to community transmission, especially in densely populated urban slums and refugee camps. The stakes are higher now because drug‑resistant TB (MDR‑TB and XDR‑TB) is on the rise, forcing health systems to spend up to three times more per patient.
Current Prevention Landscape and Its Gaps
At present, the prevention toolbox includes three main pillars:
- BCG vaccination (BCG vaccine is a live‑attenuated strain of Mycobacterium bovis given at birth in most high‑burden countries. Its efficacy against adult pulmonary TB ranges from 0-70%.)
- Tuberculosis preventive therapy (TPT) - typically a 6‑month course of isoniazid or a 3‑month weekly regimen of isoniazid‑rifapentine.
- Screening via chest X‑ray or sputum microscopy, often supplemented by the GeneXpert MTB/RIF platform (GeneXpert is an automated PCR test that detects TB DNA and rifampicin resistance within two hours.).
These tools save lives but fall short in three ways:
- BCG doesn’t protect adolescents and adults, who are most responsible for transmission.
- TPT adherence is low; many patients stop after a few weeks because of side effects or pill fatigue.
- GeneXpert, while rapid, still requires electricity, cartridge supply chains, and trained staff - a challenge in remote clinics.
Next‑Generation Vaccines on the Horizon
Scientists are betting on subunit and viral‑vector vaccines that target specific TB antigens. The most promising candidate so far is the M72/AS01E vaccine (M72/AS01E vaccine combines a fusion protein (M72) with the adjuvant AS01E to boost immune response.). A 2023 phase‑2b trial in Kenya showed a 49% reduction in active pulmonary TB among adults with latent infection.
Other pipelines include:
- Adenovirus‑based TB vaccines that stimulate mucosal immunity.
- mRNA platforms, leveraging the same technology that powered COVID‑19 shots, now in early‑stage trials.
- BCG revaccination strategies that aim to boost waning immunity in teenagers.
If phase‑3 data confirm efficacy, health ministries could roll out a single‑dose vaccine for adolescents and high‑risk adults, cutting transmission dramatically.

Rapid, Point‑of‑Care Diagnostics for Prevention
Speed matters. The longer you wait to know who’s infected, the farther the bacteria travel. Emerging CRISPR‑based tests (CRISPR‑based diagnostics use Cas enzymes to bind TB DNA and produce a fluorescent signal in under 30 minutes.) promise laboratory‑level sensitivity on a handheld device.
Imagine a community health worker walking door‑to‑door with a battery‑powered reader that tells you if you harbor active TB or a high‑risk latent infection. Coupled with mobile apps, results can be instantly uploaded to national TB registers, triggering automated contact‑tracing alerts.
Other noteworthy advances:
- Loop‑mediated isothermal amplification (LAMP) assays that work without a thermocycler.
- Breath‑omics sensors that detect volatile organic compounds specific to Mycobacterium tuberculosis.
These tools shift screening from hospitals to schools, workplaces, and refugee camps.
Digital Adherence Technologies (DATs) for Preventive Therapy
Even the best vaccine is useless if people don’t complete preventive therapy. Digital adherence technologies are closing that gap. Examples include:
- SMS reminders and two‑way messaging platforms that check in with patients daily.
- Electronic medication monitors (like the Wisepill device) that log each bottle opening and send alerts to clinicians.
- Video‑observed therapy (VOT) where patients record themselves taking medication on a secure app.
Studies from Brazil and South Africa show VOT can improve completion rates from 58% to over 80% for a 3‑month TPT regimen. The key is integrating these data streams with national TB information systems, a move many ministries are already piloting.
Policy, Funding, and the WHO End TB Strategy
Technical breakthroughs need a policy backbone. The WHO End TB Strategy (2023‑2030) sets three pillars: integrated patient‑centred care, bold policies, and intensified research. Funding gaps remain, however - the Global Fund estimates a $13billion shortfall for 2025‑2030.
Countries that align national budgets with the End TB pillars are unlocking financing mechanisms such as:
- Performance‑based grants that reward reductions in incidence.
- Public‑private partnerships for vaccine manufacturing, exemplified by the TB Vaccine Alliance.
- Innovative financing like social impact bonds for digital adherence programs.
Policymakers also need to address social determinants - overcrowding, malnutrition, and HIV co‑infection - because prevention is only as strong as the environment it operates in.
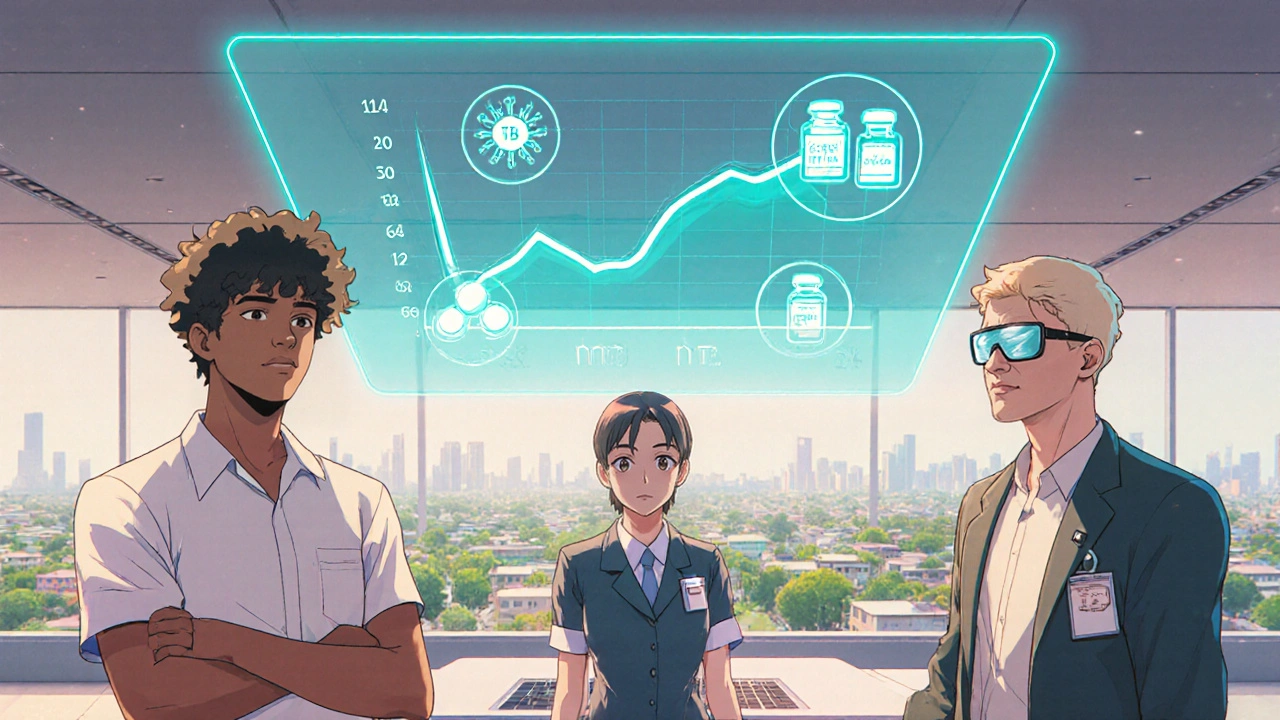
What Patients Can Expect in the Next Five Years
For the average person living in a high‑burden region, the future could look like this:
- At age 12-14, you receive a single dose of the M72/AS01E vaccine during a school health check.
- Within a year, a community health worker offers a rapid CRISPR test at your local market. If the test is positive for latent infection, you get a 3‑month TPT regimen packed in a smart pill bottle.
- Every time you open the bottle, the device sends a silent ping to your clinic. Missed doses trigger a friendly SMS reminder.
- Should symptoms appear, a handheld GeneXpert‑style device can diagnose active TB on site, allowing same‑day treatment.
This seamless flow reduces stigma, cuts out weeks of waiting, and dramatically lowers the chance you’ll spread TB to family or coworkers.
Comparison: Current vs. Future Prevention Tools
| Aspect | Current Standard | Future (2025‑2030) |
|---|---|---|
| Vaccine | BCG (single dose at birth) | M72/AS01E (single dose for adolescents/adults) |
| Preventive Therapy | 6‑month isoniazid or 3‑month weekly I/R | 3‑month daily rifapentine‑based regimen + DAT monitoring |
| Diagnostic Speed | Sputum microscopy (2‑3 days) or GeneXpert (2 hrs) | CRISPR‑based point‑of‑care (<30 min) |
| Adherence Support | Directly observed therapy (clinic‑based) | Video‑observed therapy, smart pill bottles, SMS reminders |
| Implementation Cost (per person) | $30‑$50 (BCG + TPT) | $40‑$70 (new vaccine + digital tools) |
Next Steps for Health Workers and Decision‑Makers
- Map high‑risk populations using GIS data and prioritize them for M72/AS01E trials.
- Invest in portable CRISPR devices; negotiate bulk pricing with manufacturers.
- Integrate DAT data streams into existing TB registers to flag non‑adherence in real time.
- Secure funding through the Global Fund’s “Innovative Financing” window, emphasizing cost‑effectiveness of combined vaccine‑diagnostic bundles.
- Launch community education campaigns that demystify new vaccines and highlight the ease of rapid testing.
Frequently Asked Questions
How effective is the M72/AS01E vaccine compared to BCG?
In a large phase‑2b trial, M72/AS01E reduced the risk of active pulmonary TB by about 49% among adults with latent infection, whereas BCG’s protection wanes after childhood and can be as low as 0% in adults.
Can CRISPR diagnostics be used in remote clinics without electricity?
New CRISPR platforms are battery‑operated and require only a small heating pad, making them suitable for off‑grid settings. Field trials in Bangladesh have shown reliable results with a solar‑charged unit.
What is the role of digital adherence technology in preventive therapy?
DATs-like smart pill bottles and video‑observed therapy-track each dose taken and instantly alert health workers if a patient misses a dose. This real‑time feedback has boosted completion rates from roughly 60% to over 80% in several African pilots.
Will the new TB prevention tools be affordable for low‑income countries?
While the upfront cost per person is slightly higher ($40‑$70 versus $30‑$50 for current methods), the long‑term savings from fewer active cases and reduced drug‑resistance treatment outweigh the expense. International donors are already earmarking funds for vaccine roll‑outs and point‑of‑care diagnostics.
How does HIV co‑infection affect TB prevention strategies?
People living with HIV have a 20‑30% higher risk of progressing from latent to active TB. For them, preventive therapy is especially crucial, and newer short‑course regimens combined with DATs have shown higher adherence and fewer drug‑interaction problems.


9 Comments
We keep hearing about global TB targets while the true cure lies in a fierce commitment to our own borders, a notion that seems to be lost on the world’s bureaucrats.
If America puts its money where its mouth is, we could fund home‑grown vaccine trials and outpace the rest.
The current BCG routine is a relic, and the new M72/AS01E looks promising if we give it the political will.
Still, I’m tired of the endless policy papers that never translate into action.
What we need is straight talk and real investment, not another UN proclamation.
America can lead the charge, especially when we throw our own dollars into fast‑track vaccine pipelines 🇺🇸🚀.
Our tech sector already makes mRNA shots faster than any other nation, so why wait for WHO approval?
Let’s push for domestic CRISPR‑based point‑of‑care kits and show the world how it’s done 👍.
Just looking at the US TB numbers, they’re not as low as we brag about; there are still pockets in prisons and homeless shelters where the disease spreads like gossip.
We have the labs, the data, and the people to roll out a rapid test in a community clinic tomorrow.
If we pair that with short‑course preventive therapy, the drop in cases could be measurable within a year.
The tragedy is that we treat TB as a statistical nuisance rather than a moral failing of our health system.
Every missed dose, every delayed diagnosis, is a silent indictment of our collective indifference.
We must adopt a philosophy that sees prevention as a sacred duty, not a budget line item.
Otherwise, the future we dream about will remain a fantasy built on sand.
Only a radical shift in mindset can convert data into decisive action.
Imagine walking into a school gym in 2027 and being handed a tiny pimple‑sized vaccine that promises half‑life protection against TB.
Will the children trust the needle, or will they ask the same questions we’re asking now about long‑term safety?
The blend of CRISPR diagnostics and digital adherence tools sounds like science fiction, yet the papers are already on the table.
Can we afford to wait until the next generation grows up to see the devastation we could have prevented?
These are the moments that demand our attention.
Well, let me just unpack the whole cascade of implications that most of us gloss over in a single paragraph of a policy brief, because I can’t help but feel that the sheer magnitude of this endeavor deserves a narrative that stretches beyond the bullet points that the WHO so neatly packages for us to skim over.
First, when we talk about the M72/AS01E vaccine, we are not merely discussing an antigenic formulation; we are confronting a societal contract where the government, the pharmaceutical companies, and the individual all shoulder a piece of the risk‑reward equation, and that contract is often written in language that the layperson can’t decipher, which in turn fuels mistrust and hesitancy that ripple through entire communities, especially those already marginalized by poverty and lack of access to care.
Second, the CRISPR‑based diagnostics you so enthusiastically champion, while technically elegant, rely on a supply chain for reagents and a maintenance schedule for the handheld devices that is precarious at best in regions where electricity is intermittent and the cold chain is more myth than reality.
Third, the digital adherence technologies, though praised for their sleek interface and promise of real‑time monitoring, raise profound questions about privacy, data ownership, and the psychological burden placed on patients who feel constantly watched by an invisible algorithm that logs each pill bottle opening as if it were a transaction on a stock market.
Furthermore, the notion of integrating these data streams into national TB registries sounds like a bureaucratic dream, yet in practice it often becomes a labyrinth of inter‑agency agreements, each with its own set of protocols, legal constraints, and budgetary limitations that can stall even the most well‑intentioned pilot projects for years.
Now, consider the social determinants you briefly mentioned-overcrowding, malnutrition, HIV co‑infection-these are not peripheral factors that can be patched by a vaccine shot; they are structural inequities that require sustained political will, cross‑sector collaboration, and, frankly, a reallocation of resources away from short‑term political wins toward long‑term health infrastructure building.
In addition, the financial shortfall of $13 billion projected for 2025‑2030 is not just a number; it translates into fewer training programs for community health workers, reduced capacity for local labs to adopt new technologies, and a slower rollout of performance‑based grants that could otherwise incentivize regional declines in incidence.
Even the promising performance‑based grants, while innovative, risk creating perverse incentives where success is measured by reported case reductions rather than actual health outcomes, potentially encouraging under‑reporting or manipulation of data.
Moreover, the social impact bonds aimed at funding digital adherence programs sound like a clever partnership, but they hinge on the assumption that investors will see a return, which in the volatile landscape of global health funding is far from guaranteed.
All of this means that the roadmap you present, though inspiring, is riddled with fault lines that require more than scientific breakthroughs-they demand thoughtful governance, community engagement, and a willingness to confront uncomfortable truths about inequality.
Thus, as we stand at the cusp of what could be a paradigm shift in TB prevention, we must resist the temptation to view technology as a silver bullet, and instead adopt a holistic approach that weaves together vaccine innovation, diagnostic accessibility, health system strengthening, and social justice.
Only then can we hope to truly make TB a thing of the past, rather than a recurring footnote in the annals of public health.
Colleagues, the progress outlined here reflects a convergence of scientific rigor and compassionate public health.
By aligning vaccine development with community‑driven delivery models, we nurture both immunity and trust.
The integration of CRISPR diagnostics offers a tangible pathway to early detection, which, coupled with digital adherence platforms, can dramatically improve treatment outcomes.
It is essential that policymakers allocate resources not only for technology acquisition but also for capacity building among frontline workers.
With sustained commitment, the vision of a TB‑free future becomes an attainable goal rather than a distant ideal.
totally agree, we just need more fundz and real action.
Picture a sunrise over a bustling market where no child clutches a cough, where every breath is a promise of health-this is the drama of hope we can script together.
When vaccines, rapid tests, and caring nurses unite, the battle against TB transforms from a grim saga into a triumphant anthem.
Let us raise our voices, fund the research, and lift the barriers so that every community can step into that bright new chapter.